This is a topic that I have been continuously exploring for the past 4 years. It all started out with a question that I had with an attending whether patient has pain under general anesthesia. It is a fundamental question but a key question that laid foundation to pain management.

VAS score was compared in awake, moderate MAC, deep MAC when same noxious stimulation was applied to elicit same autonomic nervous response. Subjects who received moderated and deep MAC had significant VAS score compared to awake subjects.
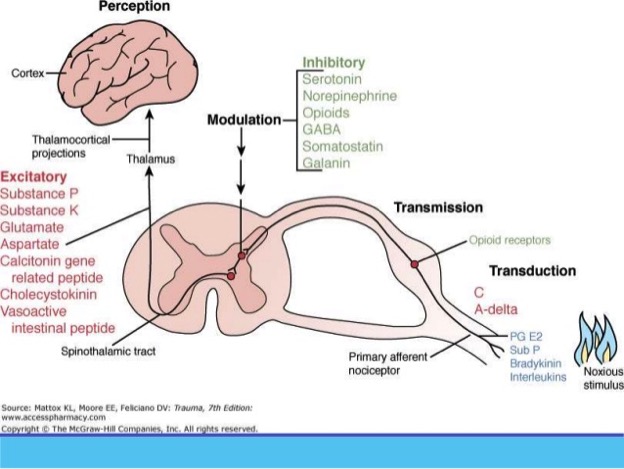
Based on the definition of pain, it is “An Unpleasant Sensory and Emotional Experience Associated with Actual or Potential Tissue Damage, or Described in Terms of Such Damage”. We can see perceiving pain requires higher function of the brain and it is the brain that is actively involved in interpreting pain intensity. For instance, British Medicine Journal reported a case that a construction worker went to ED to seek medical treatment as there was a 15cm nail penetrating through his right boot. He was experience excruciating pain from the injury. He received significant amount of opioids but the pain was not relieved. Later the nail was pulled out from below. They found out that he was not injured. The nail went through between his toes. However, his brain tricked him and made him think that he was injured and hence the pain. Another case study was a 84 years old veteran who took a xray for his routine checkup. They found a bullet in his neck which has been there for over 4 decades. He didn’t experience pain because at the time of injury in the battle field, it was more important to run for his life than worrying about his injury. That is exactly what his brain did. As we can see, pain is a protective mechanism and it is not directly correlated with the degree of tissue injury. For instance, brain can produce pain even without any physical injury. Now we know patients who are under general anesthesia do not perceive pain. Their responses to noxious stimuli are controlled by SNS, spinal cord reflex and lower brain. This notion is supported by a recent study that assessed VAS in awake, moderate MAC and deep MAC with same stimulation. The authors found that patients with sedation had lower VAS score based on the stimulation response.

The pain state has four components including somatomotor, somatosensory, autonomic and neuroendocrine systems. Somatosensory system is where we target to provide analgesia. However, the autonomic nervous system is the one system we may not tackle to treat noxious stimulation or postop pain. SNS based on the anatomy, is also called throaco-lumbar SNS. Its afferent fibers in SNS supplies visceral organs, called visceral afferent. It may serve as visceral nociception. This notion is supported by animal and RCT studies on using esmolol in reducing pain perception. For instance, multiple studies have shown the efficacy of using esmolol in abdominal, lower extremity and ENT surgeries in reducing opioid consumption intraop as well as postop. In animal studies, continuous infusion of esmolol significantly reduced the pain perception of pain when mice were put on the heated plate or injected with formalin. All of the studies suggest that there is a role of SNS in pain perception. Furthermore, most of the hemodynamics response during laparoscopic appendectomy and cholecystectomy is caused by SNS in response to insufflation (not parasympathetic response). Hence, it is visceral response through SNS system. Hence, attenuating SNS would be a perfect choice. Most of patients are comfortable with ketorolac and IV Tylenol if given at the end of the case especially if surgeons give local at the port sites.

Rent meta analysis on nociception monitoring suggested over use of opioid administration when pain is not perceived. The responses are due to sympathetic activation in response to noxious stimuli. Hence, other adjuncts such as alpha2 agonist clonicdine, precede besides beta1 antagonist would be also good choices to reduce intraop and postop pain. Some people may argue that it may cause wind-up and central sensitization if pain is not treated. Again, frontal cortex needs to be involved to initiate the central sensitization. Wind-up requires repetitive stimulation over a critical level in spinal cord neuron. Hence, patient is anesthetized under GA and noxious stimul would not be over critical level if patient is receiving adequate anesthesia when patient is under GA.
This is a long post. The take home message is that patient does not need too much opioid intraoperatively. There are other adjuncts to use to treat noxious stimuli. Hyperalgesia, delayed emergence, PONV, and impaired immunity may be minimized when minimizing opioids. However, when they are awake, patients need adequate pain control.
This makes a lot of sense. I have a question about wind-up/central sensitization. Why do you say that wind-up requires the frontal cortex? Doesn’t this process occur in the spinal cord? And if we treat the sympathetic response to noxious stimuli with sympatholytics, is the spinal cord still receiving repeated stimuli that can precipitate central sensitization? It seems that we are blocking the evidence of the stimuli at the end organ (heart), though the spinal cord neuron is still being repeatedly stimulated if we are not also taking measures to block transmission. Thank you stimulating some thought!
LikeLike
Hi Jay, thank you for reading my post. It is a great question on the windup and central sensitization. I had the same question when I first started out on exploring this topic. The windup occurs in the spinal cord. However it requires the pain stimulation to surpass a threshold to trigger the windup syndrome. It could happen when patient is not adequately anesthetized. We are not blocking the evidence of the stimuli. ANS is part of the visceral pain receptor. Hence, you are inhibiting the visceral pain pathway. In addition, when patient is under general anesthesia, nociceptive stimulation is significantly diminished as generalized activation of GABA dampen painful stimulation. This notion is supported by the most recent publication in Neuron: “A brainstem-spinal cord inhbitiory circuit for mechanical pain modulation by GABA and enkephalins.” Central sensitization is not equivalent to windup syndrome. Central sensitization requires awake brain and it depends on the synaptic plasticity in response to increased membrane excitability and decreased inhibitory pathway in nociceptive pathway. People who are more anxious are more likely to develop central sensitization in the high risk surgeries than their counterparts. In summary, we are not blocking the manifestation of the painful stimulation. GA and esmolol have shown to reduce nociceptive stimulation. Wind up requires reaching a threshold. Central sensitization is a slow process and requires awake patients to interpret the pain sensation. I hope this helps.
LikeLike